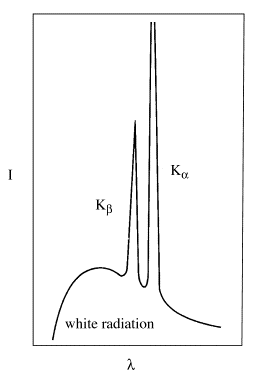
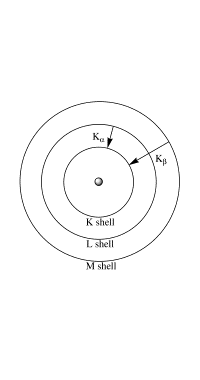
Some of the electrons are simply “slowed down” by the target; this energy is “lost” as continuous wavelength white radiation. Some electrons eject core electrons from the target atoms. When an electron from a higher energy level falls back into the lower energy vacancy, X-rays with a well-defined wavelength corresponding to the difference in energy between the two levels are produced in a direction approximately perpendicular to the electron beam. The Kα and Kβ spikes in the X-ray emission spectrum (left figure) correspond to two such energy level transitions (right figure):
 |
 |

