If the results for all three reflections are superimposed:
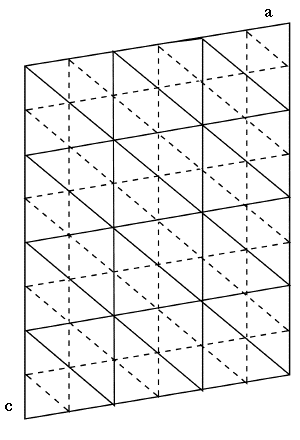
|
Again, solid lines locate electron density maxima when the phase is 0° (+|Fhkl|) and
dashed lines locate electron density maxima when the phase is 180° (-|Fhkl|). Where
three of these maxima intersect, there must be a large amount of electron density, especially if the
amplitudes of the three waves are all large (large |Fhkl|). At these triple intersection
points, in fact, there must be an atom! This is a general relationship and can be applied to other reflection triplets: |

