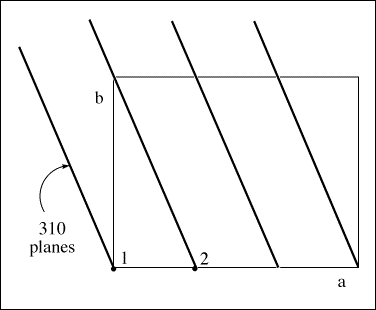
The phase difference between successive Bragg planes is 2π radians or 360°. For an atom
translated one unit cell length in the a-direction, i.e., from x = 0 to x = 1, the phase difference would be 2πh radians. For the 310
planes below, this amounts to a phase difference of 2π(3) = 6π radians.

