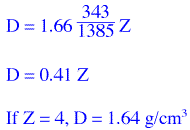As an example of a heavy atom structure, the substituted benzophenanthrene:
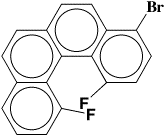
crystallizes in the monoclinic space group P21/c with cell constants
a = 7.312(2)Å, b = 13.621(3)Å, c = 13.908(3)Å, β = 90.55(2)°.
The molecular formula is C18H9F2Br; the molecular weight is 343 g/mol; the volume
of the unit cell is 1385 Å3. A preliminary estimation of the crystal density can be made using
the expression:
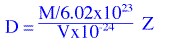
where M is the molecular weight, V is the unit cell volume, M is divided by Avogadro's No. to get the
mass of one molecule, V is multiplied by 10-24 in order to convert Å3
to cm3 and Z is the number of molecules in the unit cell. The expression reduces to
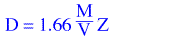
and substitution of these values for M and V produces a density estmate
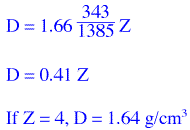
This result seems reasonable; the densities of most normal organic and inorganic compounds are
usually between 1.0 g/cm3 and 1.8 g/cm3.