Compound 9854 (Whole Molecule Disorder)
The Direct Methods solution for this structure looks “messy”. There seem to be “too many” atoms. The situation looks even worse if the Q-peaks are connected with “bonds” (click on “Bonds”). To remove bonds, click on “Clear”.
There is one feature that is apparent - a six-membered ring defined by Q4-Q17-Q6-Q20-Q13-Q19 (click “6-ring 1”). From here, it's relatively straightforward to complete this molecule (click on “molecule 1”). There must be two different orientations of the molecule superimposed. In order to differentiate the assigned peaks from the unassigned peaks, click on “yellow” to color the remaning peaks yellow.
The second molecule appears to have a six-membered ring defined by Q32-Q29-Q30-Q4-Q23-Q26 (click on “6-ring 2”). Now the remainder of this molecule can be identified by clicking on “Molecule 2”.
It is interesting that molecule 1 is constructed from the twenty top Q-pks. The peaks are numbered from highest to lowest peak size (electron density). Molecule 2 results from the smaller peaks. This implies that Molecule 1 constitutes a larger proportion of the macro-structure than Molecule 2. There are several Q-peaks that, apparently, arise from pairs of atoms in the two molecules overlapping, or nearly overlapping; for example, Q2, Q3, Q4, Q5, Q6, and Q9. These “overlapping” atoms were eventually resolved during refinement. Clearly, Q1 is the Chlorine; Q7, Q10, Q22, and Q24 are Nitrogens.
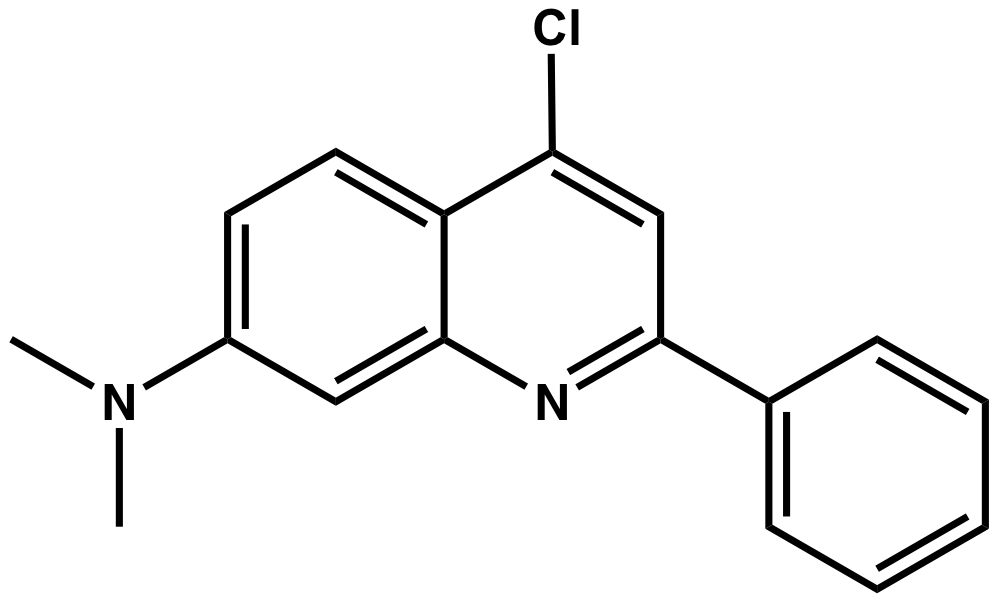
The resulting model can be displayed by clicking “Model”
In order for the two contributing disorder structures to add up overall to one molecule, the occupancy of each atom needs to be adjusted as shown in the following line from the SHELXL .res file:

In this example, the occupancy is 0.5 and has 10.0 added to it to indicate that the occupancy will not be refined.


