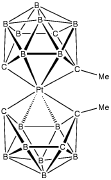
An investigation of the bonding properties of the [nido-6-Me-5,6,9-C3B7H9]- tricarbadecaboranyl monoanion included the X-ray structure determination of the Platinabis(tricarbadecaboranyl) sandwich compound:

A preliminary crystal investigation indicated the
monoclinic space group P21/c with a=17.898(3), b=10.948(1), c=14.127(2) Å,
b=108.12(3), V=2631(2)Å3. This cell
implied that there are 6 molecules in the unit cell in order to produce
a reasonable density for this compound!
The solution of the structure revealed that there are two isomers of the desired compound co-crystallized in the unit cell: one isomer has two cages of the same enantiomeric type, S-Pt-S or R-Pt
-R; one isomer has two different enantiomeric forms of the anion, R-Pt-S:
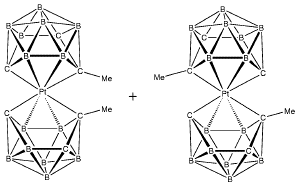
This second isomer is, in addition, centrosymmetric and lies on a crystallographic center of symmetry. Thus, there are four molecules of the S-Pt-S (R-Pt-R) configuration in the unit cell and two molecules of configuration R-Pt-S.
Below is a
ChemSymphony depiction of the packing diagram with the centrosymmetric molecules
in green and the non-centrosymmetric molecules in blue. If the cell is rotated to
view it parallel to the b-axis, the six molecules in the unit cell can be most
easily seen (To rotate the cell in the frame below, depress the mouse button
and drag in the frame.)
The Titanium cage compound shown below crystallizes in the triclinic space group P1- with a=9.342(2), b=10.051(2), c=8.4976(14) Å, a=94.83(1), b=107.35(1), g=89.78(1)°, V=758.7(2) Å3 and Z=2.
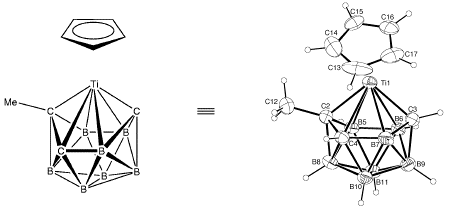
Inspection of the three-dimensional structure of the molecule shows that the Ti atom seems to have a vacant co-ordination site (depress the mouse button in the frame below and drag, to rotate the molecule):
In fact, the Ti atom is weakly co-ordinated to H5 and H6 on a neighboring molecule forming a weakly bound dimer that lies on a crystallographic center of symmetry. To interact with the "dimer" molecule in "real time", go here.