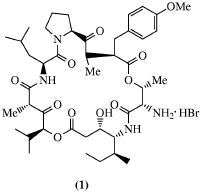
| Didemnins are a group of marine natural products which exhibit a broad spectrum of biological activities. An X-ray crystal structure analysis of the hydrobromide, (1), which contains the common cyclic core of didemnins was performed on a sample synthesized by the Joulliť research group. Analysis of this compound and natural didemnin B revealed some conformational discrepancies which may be responsible for the striking difference in biological activities. |  |
Below are ORTEP representations of the two molecules in the asymmetric unit showing intramolecular hydrogen bonds :
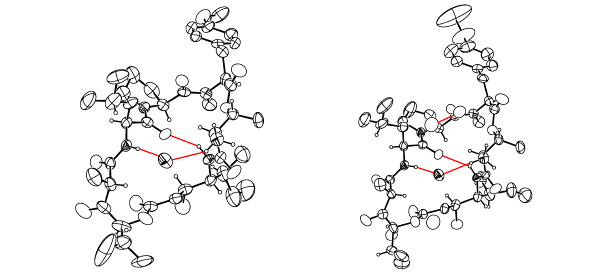
To perform real-time measurements on this molecule go here.