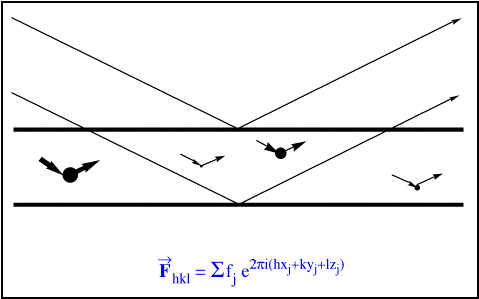
The picture of Bragg's Law and that simple planar molecule in
the Introduction certainly oversimplifies the situation: in general, the electron densities in the
unit cell are not concentrated in parallel planes. A more realistic depiction would have atoms
dispersed at various locations in between those planes:


