An ORTEP drawing of the
molecule at the first level of approximation shows slightly larger atom representations for those
atoms which would be expected to have greater thermal motion (e.g., the various methoxy groups and
the t-butyl group):
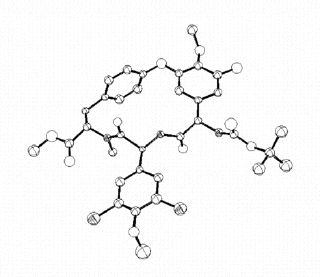
This first approximation, which uses a single temperature factor for each atom, allows the atoms to vibrate, but with equal magnitudes in all three directions. Thus, the single B factor is called an isotropic temperature factor.
There is a more experimentally reasonable approximation
(anisotropic) which allows each atom to move a different amount in the three different co-ordinate
directions:
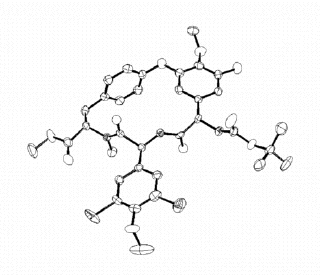
An ORTEP with anisotropic temperature factors shows, for example, that the methoxy carbon atom at the “bottom” of the molecule probably experiences a greater amount of vibration due to bending of the O-Me bond than stretching of that bond.

